X Chromosome Inactivation (XCI) is a fascinating biological process that plays a critical role in balancing gene expression between the sexes. In females, who possess two X chromosomes, one copy must be silenced to prevent an overload of gene dosage, a mechanism vital for normal development. This chromosomal silencing is intricately controlled by Xist RNA, which ensures that one of the X chromosomes remains inactive while the other remains functional. Understanding the dynamics of XCI is essential, particularly in the context of genetic diseases such as Fragile X Syndrome and Rett Syndrome, which are linked to mutations on the X chromosome. Recent research into XCI not only sheds light on fundamental cellular processes but also opens exciting avenues for potential therapies targeting these debilitating conditions and beyond.
The phenomenon of X Chromosome Inactivation (XCI), sometimes referred to as X-linked gene silencing, serves as a vital mechanism in female cells to maintain genetic balance. In essence, females carry two copies of the X chromosome, leading to a need for one to be inactivated to ensure proper gene expression levels. This intricate process is governed by specialized RNA molecules, particularly Xist RNA, which orchestrate the silencing of one X chromosome. Such chromosomal regulation becomes especially significant when considering various genetic disorders associated with the X chromosome, including notable conditions like Fragile X Syndrome and Rett Syndrome. Therefore, deciphering the complexities of XCI promises not only to enhance our understanding of these genetic diseases but also to lay the groundwork for innovative therapeutic strategies.
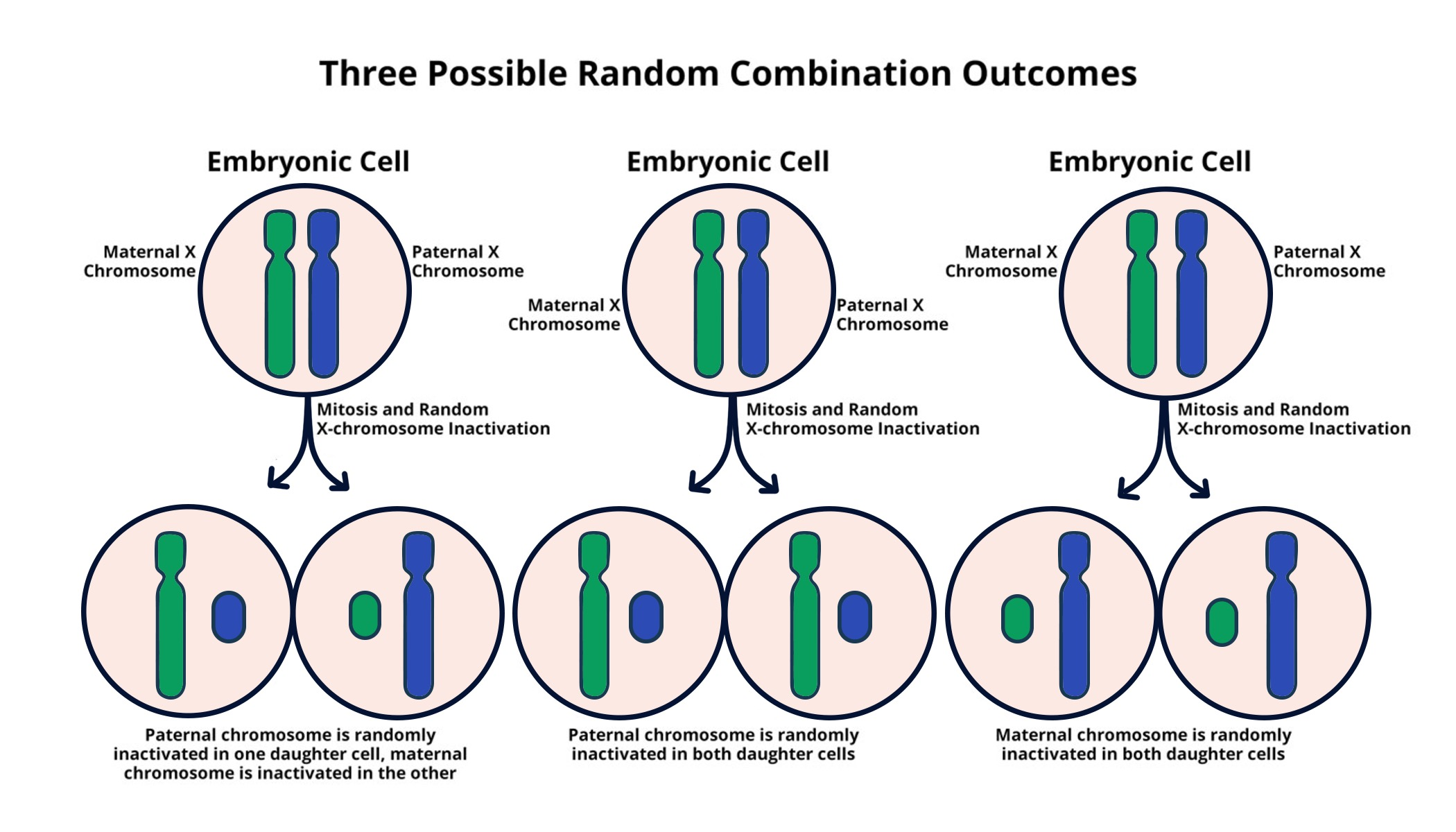
Understanding X Chromosome Inactivation
X chromosome inactivation (XCI) is a vital biological process that occurs in females, who have two X chromosomes compared to males with one. This unique mechanism ensures that gene dosage remains balanced between sexes, as having two active X chromosomes would produce an excess of gene product, potentially leading to developmental issues. The process involves the silencing of one X chromosome, allowing only one to remain active. At the molecular level, this intricate choreography involves the Xist RNA molecule, which plays a pivotal role in initiating the inactivation process by coating the chromosome and altering the local chromosomal environment.
Recent advancements in our understanding of XCI shed light on the complexities of chromosomal silencing. Research by scientists like Jeannie T. Lee has unraveled how Xist RNA interacts with the adjacent chromatin, modifying its biophysical properties, which facilitates the inactivation of the chromosome. This transformation of the chromatin structure creates a more flexible environment, enabling the efficient recruitment of various silencing factors that contribute to the eventual inactivation of the chromosome. As these discoveries unfold, they pave the way for therapeutic applications targeting genetic diseases associated with the X chromosome.
The Role of Xist RNA in Genetic Diseases
Xist RNA is central to the regulation of X chromosome inactivation. Its mechanism not only exemplifies a fascinating aspect of gene regulation but also highlights its implications in the context of genetic diseases, particularly those linked to the X chromosome such as Fragile X Syndrome and Rett Syndrome. Mutations in genes located on the X chromosome can lead to severe developmental disorders, often because one copy is dysfunctional while the other remains inactive and unavailable for gene expression. Understanding how Xist RNA triggers inactivation thus becomes crucial in both understanding and potentially reversing the effects of these genetic disorders.
The therapeutic potential for manipulating X chromosome inactivation is promising, particularly with emerging techniques to unsilence genes that are currently dormant due to XCI. By using compounds that target Xist RNA or alter the chromatin environment, researchers like Lee are exploring ways to reactivate the healthy gene on the silent X chromosome. This could lead to groundbreaking treatments for Fragile X and Rett syndrome, providing hope for numerous affected individuals. The ongoing research aims to optimize safety and efficacy, potentially moving towards clinical trials that could change lives.
Implications for Fragile X and Rett Syndromes
Fragile X Syndrome and Rett Syndrome are two neurodevelopmental disorders linked to mutations on the X chromosome, highlighting the importance of understanding XCI mechanisms in developing targeted therapies. Fragile X Syndrome is characterized by cognitive impairment and behavioral challenges, primarily affecting males due to its location on the X chromosome. Similarly, Rett Syndrome predominantly impacts females and leads to loss of acquired skills and severe impairments. Both conditions exemplify how mutations on one X chromosome can lead to profound challenges, particularly when the healthy gene is inactive due to XCI.
With new insights from studies on X chromosome inactivation, there is cautious optimism regarding treatment strategies that could potentially restore function to the affected genes. By addressing the inactivation issues directly, researchers are devising experimental approaches that may enable the selective reactivation of the healthy X-linked genes in individuals with these syndromes. This could offer a dual benefit: improving symptoms associated with the genetic disorder while maintaining the overall stability of X-linked gene expression. Such advancements could lead to viable therapies for conditions previously deemed hard to treat.
Chromosomal Silencing and Genetic Disease Treatment
Chromosomal silencing plays a significant role in maintaining genetic balance but can also present unique challenges when it comes to treating genetic diseases, especially those linked to the X chromosome. Research conducted by Jeannie T. Lee’s lab gives deeper insight into how this silencing occurs and highlights its implications for therapy. The gel-like substance that surrounds chromosomes, enabling the silencing process, acts as a barrier that can inadvertently keep beneficial genes inactive, thus contributing to the pathology of various genetic disorders.
By targeting the mechanisms of chromosomal silencing, scientists hope to devise innovative therapies that can unsilence these dormant genes and restore their function. The potential to alter the properties of the chromosomal environment, particularly through manipulation of Xist RNA, opens up new avenues in gene therapy, particularly for individuals affected by conditions such as Fragile X and Rett syndromes. As researchers refine these techniques, the prospect of transitioning these findings from bench to bedside becomes increasingly tangible, triggering a new era of treatment for X-linked genetic diseases.
Future Directions in X Chromosome Research
The research into X chromosome inactivation and its therapeutic implications is in a progressive phase, with scientists continuously seeking to unravel the complexities surrounding this fundamental biological process. Future directions in this field will likely focus on fully elucidating the molecular players involved in XCI and how they can be harnessed for treatment. As new technologies emerge, such as gene editing and RNA therapies, researchers are poised to develop innovative strategies that could enable the selective targeting and modulation of X-linked gene expression.
Furthermore, understanding the nuances of chromosomal silencing will enrich our knowledge of not only what’s transpiring in genetic diseases but also how broader mechanisms of gene regulation operate. This multi-faceted exploration might unveil additional chromosomal interactions and their role in other genetic disorders, thus paving the pathway for developing novel therapeutic modalities. The continual collaboration between basic research and clinical application is essential in translating these scientific discoveries into meaningful treatments for patients suffering from genetic conditions associated with the X chromosome.
The Importance of Gene Dosage and Balance
Gene dosage refers to the number of copies of a particular gene present in a cell, and it plays a critical role in gene expression and cellular function. In the context of X chromosome inactivation, maintaining gene dosage balance between males and females is crucial to avoid the overexpression of genes located on the X chromosome, which can disrupt normal cellular processes. This balance is meticulously regulated through XCI, which not only silences one of the two X chromosomes in females but also prevents potential imbalances in gene output that could lead to various disorders.
Any disruption in this gene dosage balance can lead to alterations in phenotype, as seen in disorders like Fragile X Syndrome. The complex nature of chromosomal silencing through processes like XCI is therefore instrumental in ensuring that females do not express two full sets of X-linked genes simultaneously. By understanding how these mechanisms ensure proper gene dosage, researchers can develop targeted strategies to restore normal gene balance for individuals suffering from X-linked conditions, leading to effective therapeutic interventions.
Challenges in Gene Therapy for X-linked Disorders
While the potential for gene therapy to treat X-linked disorders is promising, several challenges remain. One of the central concerns is ensuring targeting specificity—avoiding the unintended activation of non-mutated genes while attempting to reactivate dormant ones. This specificity is crucial to prevent the disruption of already balanced gene expression levels across the genome. Furthermore, the long-standing inquiry into how exactly X chromosome inactivation functions continues to pose challenges in developing effective therapies, as a misstep could inadvertently exacerbate conditions or produce unwanted side effects.
Current approaches, including the use of Xist RNA and other key molecules, must be finely tuned to achieve the desired therapeutic outcomes. Hence, extensive preclinical trials and safety assessments are essential before transitioning to human applications. Moreover, as research progresses, refining these targets and honing delivery mechanisms will be vital to ensure that treatments for diseases such as Fragile X and Rett syndrome are effective and safe, paving the way for successful clinical implementation.
The Potential of Uncovering Genetic Therapies
The prospect of uncovering viable genetic therapies through the manipulation of X chromosome inactivation is a frontier in genetic research, promising to provide solutions for previously untreatable conditions. By understanding the mechanisms regulating XCI and leveraging them for therapeutic strategies, researchers aim to overcome the limitations presented by dormant genes on the inactivated chromosome. Such breakthroughs could revolutionize treatment paradigms for a range of genetic disorders, particularly those linked to X-linked inheritance patterns.
As scientists continue to validate their models and refine their approaches to gene therapy, the focus will increasingly target precision—ensuring that therapies not only reactivate the desired genes but also sustain the overall genetic health of the individual. This holistic view of therapy development will be critical as researchers seek to deliver effective solutions that can alleviate or even eradicate the symptoms of genetic conditions like Fragile X Syndrome and Rett Syndrome, ultimately leading to a better quality of life for patients and their families.
Broadening Horizons in Genetic Research
The ongoing exploration of X chromosome inactivation and its implications for gene expression showcases a significant leap in the field of genetics, but it also underscores the necessity for broader genomic research. While X-linked diseases provide a rich ground for study, many other genetic conditions also require innovative approaches that stem from fundamental biological understanding. By drawing connections between various genetic mechanisms—such as those governing chromosomal behavior, gene dosage, and regulatory RNA—researchers can develop comprehensive strategies to tackle multiple disorders.
In addition, the collaborative nature of modern science accelerates this progress, with shared knowledge across disciplines—such as genomics, molecular biology, and clinical medicine—fueling innovations. As technologies advance and interdisciplinary approaches become common practice, the potential to unlock the secrets of both familiar and obscure genetic conditions expands significantly. Therefore, the research surrounding X chromosome inactivation is not just pertinent to Fragile X and Rett Syndromes; it may illuminate paths to effective therapies for a myriad of genetic diseases, impacting countless lives.
Frequently Asked Questions
What is X Chromosome Inactivation and why is it important in genetics?
X Chromosome Inactivation (XCI) is a biological process in which one of the two X chromosomes in female mammals is inactivated to prevent gene dosage imbalance between genders. This process is crucial for female development and plays a significant role in the manifestation of genetic diseases linked to the X chromosome, such as Fragile X Syndrome and Rett Syndrome.
How does Xist RNA contribute to X Chromosome Inactivation?
Xist RNA is essential for X Chromosome Inactivation as it coats the X chromosome, facilitating chromosomal silencing. This RNA alters the biophysical properties of the surrounding chromosomal material, which helps in the inactivation process, effectively rendering the chromosome inactive and unavailable for gene expression.
What are the implications of X Chromosome Inactivation for diseases like Fragile X Syndrome?
X Chromosome Inactivation has significant implications for diseases such as Fragile X Syndrome. Since mutations can exist on one of the X chromosomes, inactivating the mutated chromosome could allow the healthy version of the gene on the other chromosome to function, potentially paving the way for therapeutic strategies to treat genetic disorders linked to the X chromosome.
Why is X Chromosome Inactivation more complex in females compared to males?
X Chromosome Inactivation is more complex in females because they possess two X chromosomes, while males have only one. To maintain gene dosage balance, females must inactivate one of their X chromosomes, which requires a sophisticated mechanism that involves Xist RNA and various silencing factors, unlike males who express genes from their single X chromosome.
What advancements in research are being made regarding X Chromosome Inactivation and genetic therapies?
Recent advancements in research on X Chromosome Inactivation, particularly from Jeannie Lee’s lab, suggest potential therapies for conditions such as Fragile X and Rett syndromes. These studies focus on unsilencing inactivated X-linked genes to restore function, indicating a promising direction for future clinical trials in treating these genetic diseases.
Can X Chromosome Inactivation techniques be applied to treat males with X-linked diseases?
Yes, X Chromosome Inactivation techniques may also benefit males with X-linked diseases. Although males do not undergo XCI like females, a similar silencing effect can occur if a mutation is present on the X chromosome. Developing therapies that unsilence these mutated genes could enhance their expression and possibly alleviate symptoms of associated genetic disorders.
What role does chromosomal silencing play in the context of X-linked genetic diseases?
Chromosomal silencing, particularly through X Chromosome Inactivation, plays a critical role in the expression of X-linked genetic diseases. It can sequester mutated genes from functioning, emphasizing the importance of understanding this mechanism for developing targeted genetic therapies that could reactivate critical genes and ameliorate disease symptoms.
| Key Points | Details |
|---|---|
| Overview of X Chromosome Inactivation | Females have two X chromosomes that need to inactivate one to avoid overexpression of genes. |
| Importance of Research | Research at Jeannie Lee’s lab focuses on understanding the X inactivation process, which could lead to therapies for X-linked diseases. |
| Role of Xist | The gene Xist plays a key role in coating and inactivating the X chromosome by interacting with surrounding chromosomal material. |
| Therapeutic Potential | Unlocking inactivated X chromosomes could provide cures for disorders like Fragile X and Rett Syndromes. |
| Future Directions | Ongoing optimization of therapeutic approaches and upcoming clinical trials aim to test their efficacy. |
Summary
X Chromosome Inactivation plays a pivotal role in cellular biology, particularly for females who possess two X chromosomes. This complex process ensures that one X chromosome is silenced, allowing for gene balance between sexes. Research led by Jeannie Lee and her team at Harvard Medical School has unveiled mechanisms behind this inactivation, revealing potential therapeutic avenues for genetic disorders stemming from X-linked mutations. As they advance their promising strategies towards clinical applications, the implications for diseases like Fragile X and Rett Syndromes become increasingly hopeful. This breakthrough not only enhances our understanding of X chromosome dynamics but also opens doors to innovative treatments that could ameliorate the impact of genetic diseases.